Abstract
Background: Recent literature provides conflicting views on whether there is a negative value trend in pharmaceutical oncology treatments. A previous study (J Econ Perspect 2015;29:1) found that the price of pharmaceutical oncology treatments has outpaced the improvements in survival benefits over time, with most treatments developed since 1995 offering marginal improvements in survival with significant increases in costs. In contrast, other literature suggests pharmaceutical oncology treatments have produced significant value.
Chimeric antigen receptor t-cell (CAR-T) therapy represents a novel approach for treating particular hematologic cancers-including pediatric acute lymphoblastic leukemia and diffuse large b-cell lymphoma-but a remaining question is whether CAR-T represents a continued trend of low-value innovation, or a significant break from past trends.
In this study, we investigate three key questions: (i) what are the trends over the past 10 to 20 years in incremental quality-adjusted life years (QALYs) and incremental cost per incremental quality-adjusted life year (cost/QALY) for newly approved anti-cancer innovations? (ii) how do innovations for hematologic cancers differ from those for other cancers in terms of this value trend? and (iii) how does CAR-T therapy compare with the recent history of innovations for both hematologic and non-hematologic cancers?
Methods: We identified all analyses of pharmaceutical treatments for cancer published since 2007 that appear in the Tufts Medical Center Cost-Effectiveness Analysis (CEA) Registry, a comprehensive database focusing on a subset of CEAs called cost-utility analyses (CUAs), which quantify health benefits in terms of QALYs. CUA results on CAR-T therapies came from an analysis conducted by the Institute for Clinical and Economic Review (2018). We limited the CUAs in our study to those conducted in the US context with at least one of the following measures of value: incremental QALYs or cost/QALY, and with intervention/indication approval year 1995 or later.
We measured linear trend in the value measures as a function of approval year and compared interventions for non-hematologic cancers with non-CAR-T interventions for hematologic cancers and, in turn, with CAR-T therapy. Regression analysis was used to control for the potential influence of characteristics of a CUA such as discount rate chosen, time horizon, number of years between publication and approval of the intervention for the indication in question, and an indicator for rare diseases as defined by the Genetic and Rare Diseases Information Center of the NIH. Indicators for CAR-T therapy and for non-CAR-T treatments for hematologic cancers were included to test for differences from innovations for other cancer types.
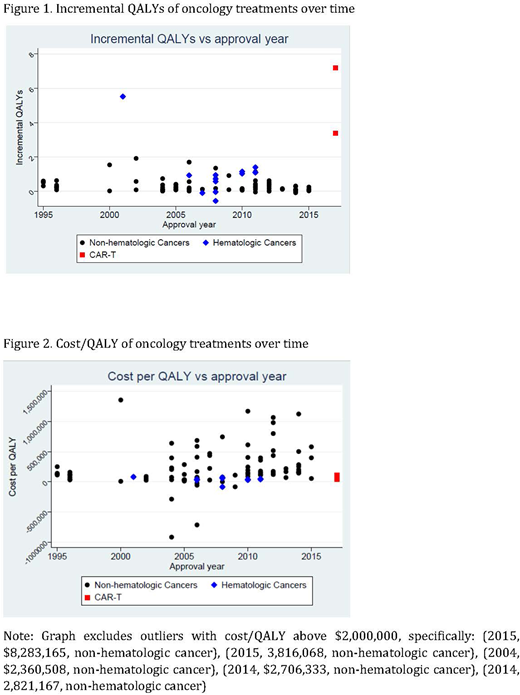
Results: 103 incremental QALY and 108 cost/QALY measures met our inclusion criteria. The figures show incremental QALYs and cost/QALY, respectively, by approval year. The regression analysis shows that -- for innovations as a whole -- incremental QALYs gained have declined with approval year by 0.079 per year (95% CI -0.128 to -0.030). Cost effectiveness has worsened somewhat over time although that trend is not statistically significant (cost/QALY increase of $36,147 per year, 95%CI -$29,575 to $101,868).
CAR-T is substantially more effective than both pharmaceutical cancer innovations outside of hematology (5.17 more incremental QALYs, 95%CI 4.09 to 6.25) and other innovations within hematology (4.67 more incremental QALYs, 95%CI 3.44 to 5.90). Innovations in hematology other than CAR-T are somewhat more effective than innovations outside of hematology (0.5 more incremental QALYs, 95%CI -0.09 to 1.09), although the difference is not statistically significant. Differences in cost/QALY between CAR-T and other pharmaceutical interventions are not statistically significant.
Conclusions: CAR-T therapy improved health outcomes (measured in QALYs) to a greater extent than both treatments for non-hematologic cancers and non-CAR-T treatments for hematologic cancers, with no significant differences in cost/QALY. Those improvements represent a break from a trend previously reported in the literature of negligible incremental effectiveness and rising costs per life-year gained.
Baumgardner:Precision Health Economics: Employment. Everson:Precision Health Economics: Employment. Brauer:Precision Health Economics: Employment. Zhang:Novartis Pharmaceuticals Corporation: Employment, Equity Ownership. Hao:Novartis Pharmaceuticals Corporation: Employment, Equity Ownership. Liu:Novartis Pharmaceuticals Corporation: Employment, Equity Ownership. Lakdawalla:Precision Health Economics: Consultancy; Novartis Pharmaceuticals Corporation: Other: PHE conducted the research underlying this study with funding from Novartis.; Precision Medicine Group: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal